No products
There are 0 items in your cart. There is 1 item in your cart.
Next order for this product:
You will be able to change this date at the next step
You will have products in your subscriptions
For a total price of (instead of ) and a delivery price of
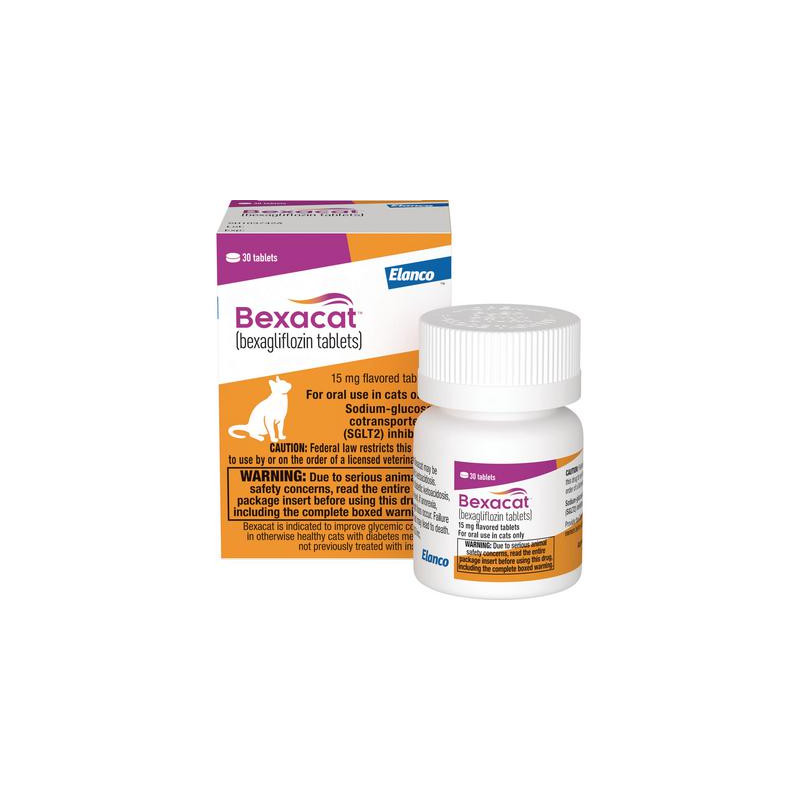
Bexacat is a once-daily tablet alternative to insulin for treating feline diabetes. It lowers blood sugar by increasing urine excretion of glucose through inhibition of sodium-glucose cotransporter 2 (SGLT2) and results in effective glycemic control. Bexacat is indicated to improve glycemic control in otherwise healthy cats with diabetes mellitus not previously treated with insulin.
Benefits:
- For oral use in cats only
- Minimal risk of hypoglycemia and no dosage changes during treatment
- Convenient, needle-free, once-daily flavored tablet ensures dosing certainty
- No dose titration needed
- No refrigeration required
- Can be given with or without food
Manufacturer:
Elanco
Active ingredient:
Bexagliflozin
Formulation:
15 mg flavored oral tablet available in 90 count size.
Contraindications:
- Do not use Bexacat in cats with diabetes mellitus who have previously been treated with insulin, who are receiving insulin, or in cats with insulin-dependent diabetes mellitus. The use of Bexacat in cats with insulin-dependent diabetes mellitus, or the withdrawal of insulin and initiation of Bexacat, is associated with an increased risk of diabetic ketoacidosis or euglycemic diabetic ketoacidosis and death.
- Due to risk of severe adverse reactions, do not use Bexacat in cats with evidence of hepatic disease of reduce renal function.
Precautions:
- Bexacat should be discontinued in cats who develop diarrhea unresponsive to conventional therapy
- Consider temporary discontinuation of Bexacat in cats during times of decreased caloric intake, such as surgery or decreased appetite, as administration of Bexacat in these cats may increase the risk of diabetic ketoacidosis or hepatic lipidosis.
- The osmotic diuretic effect of Bexacat may contribute to inappropriate urination in some cats (see Adverse Reactions)
- Polyphagia as a compensatory response to caloric wasting from glucosuria may persist in up the 80 of cats, despite evidence of adequate glycemic control, and may lead to progressive weight gain.
- Approximately 20-30 of cats may have persistent polyuria and/or polydipsia secondary to Bexacat-induced osmotic diuresis and may be a risk factor for dehydration-associated diabetic ketoacidosis.
- The safety of Bexacat in breeding, pregnant, and lactating cats has not been evaluated.
Caution:
Not for use in humans, Keep our of reach of children. Consult a physician in case of accidental ingestion by humans.
Storage:
Bexacat should be stored at tum temperature 68 too 77 °F (20 to 25 C°).
Dosage:
Administer one tablet by mouth to cats weighing 6.6 lbs (3.0 kg) or greater once daily, at approximately the same time each day, with or without food, and regardless of blood glucose level.
Monitoring:
- Sudden onset of hyporexia/anorexia, lethargy, dehydration, or weight loss in cats receiving Bexacat should prompt immediate discontinuation of Bexacat and assessment for diabetic ketoacidosis, regardless of blood glucose level.
- During treatment with Bexacat, blood glucose, fructosamine, serum β-hydroxybutyrate (BHBA), serum feline pancreas-specific lipase (fPL), liver parameters, serum cholesterol and triglycerides; and body weight and clinical signs should be routinely monitored.
- Increasing or persistently elevated feline pancreas-specific lipase or liver parameters should prompt further evaluation for pancreatitis and/or hepatic disease and consideration for discontinuing Bexacat.
- BHBA is the predominate ketoacid in diabetic ketoacidosis. Bexacat should be discontinued if a notable reduction in BHBA is not observed after initiation of Bexacat, or if BHBA persistently rises after an initial reduction.
- Cats with increasing or persistently elevated cholesterol and triglyceride levels may be at an increased risk for developing diabetic ketoacidosis or euglycemic diabetic ketoacidosis.
- Bexacat should be discontinued if poor glycemic control, as described below, develops.
- During the first 8 weeks after initiation of Bexacat, assessment of glycemic control and clinical improvement should be evaluated.
- A physical examination, an 8-hour blood glucose curve, serum fructosamine and body weight improvement should be evaluated.
- Cats demonstrating poor glycemic control, including weight loss, an average blood glucose concentration from an 8-hour blood glucose curve ≥ 250 mg/dL, and/or a fructosamine indicating poor glycemic control, as described above, at 8 weeks.
- Bexacat should be discontinued, and initiation of insulin considered in cats demonstrating poor glycemic control, as described above, at 8 weeks.
- Cats may present with diabetic ketoacidosis and a normal blood glucose concentration (euglycemic diabetic letoacidosis). Delay in recognition and treatment of diabetic ketoacidosis and euglycemic diabetic ketoacidosis may result in increased morbidity and mortality.
- Development of diabetic ketoacidosis and euglycemic diabetic ketoacidosis requires the following actions:
- Discontinuation of Bexacat
- Prompt Initiation of insulin therapy
- Administration of dextrose or other carbohydrate source, regardless of blood glucose concentration
- Appropriate nutritional support should be promptly initiated to prevent or treat hepatic lipidosis.
Active ingredient:
Bexagliflozin
- For oral use in cats only
- Minimal risk of hypoglycemia and no dosage changes during treatment
- Convenient, needle-free, once-daily flavored tablet ensures dosing certainty
- No dose titration needed
- No refrigeration required
- Can be given with or without food